"Atlas of Paleogene Cosmopolitan Deep-Water Agglutinated Foraminifera"
M.A.Kaminski and F.M.Gradstein
Preface
This book is intended to serve as a handbook for micropalaeontologists working with agglutinated benthic foraminifera found in deep marine strata of Paleogene age around the world. Our approach t o this study has been to select Deep Water Agglutinated Foraminiferal (DWAF) species that are either used in Paleogene stratigraphy, or comprise an important component of Paleogene benthic assemblages in siliciclastic sediments. The overwhelming majority of species included in the atlas are cosmopolitan, and those few that are endemic are of regional stratigraphic significance. Since a number of the taxa are stratigraphically long ranging (mainly single-chambered forms), while others appeared either in Late Cretaceous time or extend into the Neogene, the atlas in fact covers more than just the Paleogene. We have aimed at a practical approach for the atlas, with taxa grouped in natural taxonomic units. There is concise text for each of the 130 taxa that represent the majority of species to be expected in Paleogene deep marine sediments. For each of these valid species we provide type illustrations, and one or more plates with SEM and optical photographs and/or camera lucida drawings, with characteristic views of the tests in samples from several different sedimentary basins. We offer this guide to our colleagues as a pathway through the 'jungle' of intercontinental taxonomic, stratigraphic and palaeoecologic literature. Over the last two decades we have endeavoured to stabilise the classical eastern European taxonomy through translation of major studies, re-study of type collections, re-discovery of 'lost' type localities, and completion of incomplete historical taxonomic procedures. Virtually all published uppermost Cretaceous through Paleogene DWAF taxa from the European and American continents have been 'under the knife' in our laboratories. Our direct involvement in exploration micropalaeontology of many petroleum basins where DWAF are important has been of considerable assistance to this project. We only decided that we had stumbled upon some unknown new species after many years of comparative study and consultations with colleagues well acquainted with local stratigraphy and micropalaeontology.
This Atlas of Paleogene Cosmopolitan Deep Water Agglutinated Foraminifera begins with an Introduction outlining the history of investigation and lists the important collections. The second chapter introduces the main biofacies and addresses topics on palaeoecology and its spinoff, palaeobathymetry - fields that are in demand for geoscience and petroleum modelling studies of deep marine basins. Chapter three summarises the biostratigraphical record of DWAF in offshore eastern Canada, the North Sea, offshore mid-Norway, Norwegian-Greenland Sea, Barents Sea, Beaufort Sea, Carpathian flysch basins, southern European Tethyan basins, West Africa, and Trinidad/Venezuela. Each main area of investigation has a stratigraphic range chart for key taxa. The following section constitutes the main part of this atlas: Systematic Taxonomy. The taxonomic chapter is followed by the master reference listing and the species index.
This publication aims at stabilising the systematics and enhancing the economic applicability of DWAF. The study had its early roots as far back as the early 1970's. At that time, W.A. Berggren (WAB), while preparing to go to sea on Leg 12 of the Deep Sea Drilling Project, was studying agglutinated assemblages in North Sea wells, and one of us (FMG) was trying to make sense out of Cenozoic agglutinated assemblages, offshore eastern Canada. It became apparent that similar assemblages are found in these two continental margin basins, and these display general similarity t o the flysch-type faunas of the mobile tectonic belts of the Carpathian Mountains. These facts became the basis for the long-term investigation on the agglutinated benthic foraminifera, which was an outgrowth of a larger project devoted to the study of Cenozoic Deep Water Benthic Foraminifera. At its acme, this program enjoyed the support of 14 major oil companies, including ARCO, BP, Chevron, Elf-Aquitaine, Exxon, Gulf, Mobil, Marathon, Phillips, Shell (Houston), Shell International (The Hague), Statoil, Texaco, and Unocal. During the last phase of the work on this Atlas, Saga Petroleum in Norway provided vital financial assistance.
We view this study as only a first step towards a more comprehensive documentation of the global DWAF faunas through geological time. These faunas started to flourish in deep marine basins as early as Middle Jurassic, with major phases of evolutionary expansion in the Aptian-Turonian, Campanian, Paleocene, Early Eocene, and Early Miocene; the fauna is also an important constituent of bottom dwelling foraminiferal assemblages along modern continental margins. It is our wish that this atlas may be of help with the task of a more thorough and comprehensive evaluation of the taxonomy, stratigraphy and palaeoecology of this remarkably diversified and cosmopolitan group of benthic organisms that serve well in geological investigations.
Table of Contents

Taxonomic Index
No. |
Species name |
109 |
Adercotryma agterbergi Gradstein & Kaminski |
110 |
Ammoanita ingerlisae Gradstein & Kaminski |
111 |
Ammoanita ruthvenmurrayi (Cushman & Renz) |
070 |
Ammobaculites agglutinans (d'Orbigny), em. B |
071 |
Ammobaculites jarvisi Cushman & Renz |
014 |
Ammodiscus cretaceus (Reuss) |
015 |
Ammodiscus glabratus Cushman & Jarvis |
016 |
Ammodiscus latus Grzybowski |
019 |
Ammodiscus nagyi Kaminski |
010 |
Ammodiscus pennyi Cushman & Jarvis |
018 |
Ammodiscus peruvianus Berry |
020 |
Ammodiscus tenuissimus Grzybowski |
021 |
Ammolagena clavata (Jones & Parker) |
072 |
Ammomarginulina aubertae Gradstein & Kaminski |
087 |
Ammosphaeroidina pseudopauciloculata (Mjatliuk) |
029 |
Annectina biedai Gradstein & Kaminski |
028 |
Annectina grzybowskii (Jurkiewicz) |
038 |
Aschemocella carpathica (Neagu) |
039 |
Aschemocella grandis (Grzybowski) |
001 |
Bathysiphon microrhaphidus Samuel |
090 |
Budashevaella multicamerata (Voloshinova) |
091 |
Budashevaella trinitatensis (Cushman & Renz) |
073 |
Buzasina pacifica (Krasheninnikov) |
040 |
Caudammina excelsa (Dylazanka) |
041 |
Caudammina ovula (Grzybowski), em. Ge |
042 |
Caudammina ovuloides (Grzybowski) |
057 |
Conglophragmium irregularis (White) |
113 |
Conotrochammina voeringensis Gradstein & Kaminski |
114 |
Conotrochammina whangaia Finlay |
092 |
Cribrostomoides subglobosus (Cushman) |
093 |
"Cribrostomoides" trinitatensis Cushman & Jarvis |
118 |
Cyclammina cancellata Brady |
119 |
Cyclammina placenta (Reuss) |
088 |
Cystammina pauciloculata (Brady) |
089 |
Cystammina sveni Gradstein & Kaminski |
102 |
Duquepsammina cubensis (Cushman & Bermúdez) |
074 |
Eratidus gerochi n.sp. |
022 |
Glomospira charoides (Jones & Parker) |
023 |
Glomospira diffundens Cushman & Renz |
024 |
"Glomospira" glomerata (Grzybowski) |
025 |
Glomospira gordialis (Jones & Parker) |
026 |
"Glomospira" irregularis (Grzybowski) |
027 |
"Glomospira" serpens (Grzybowski) |
030 |
Glomospira(?) sp. 4 |
075 |
Haplophragmoides eggeri Cushman |
082 |
Haplophragmoides excavatus Cushman & Waters |
076 |
Haplophragmoides glabrus Cushman & Waters |
077 |
Haplophragmoides horridus (Grzybowski) |
078 |
Haplophragmoides kirki Wickenden |
079 |
Haplophragmoides porrectus Maslakova |
080 |
Haplophragmoides stomatus (Grzybowski), em. K&Ge |
081 |
Haplophragmoides suborbicularis (Grzybowski), em. K&G |
083 |
Haplophragmoides walteri (Grzybowski) |
043 |
Hormosina trinitatensis Cushman & Renz |
044 |
Hormosina velascoensis (Cushman) |
045 |
Hormosinella distans (Brady) |
046 |
Hormosinelloides guttifer (Brady) |
012 |
"Hyperammina dilatata" Grzybowski |
013 |
Hyperammina rugosa Verdenius & van Hinte |
047 |
Kalamopsis grzybowskii (Dylazanka) |
130 |
Karreriella seigliei (Gradstein & Kaminski) |
115 |
Karrerulina coniformis (Grzybowski) |
116 |
Karrerulina conversa (Grzybowski) |
117 |
Karrerulina horrida (Mjatliuk) |
058 |
Lituotuba lituiformis (Brady) |
002 |
Nothia excelsa (Grzybowski), em. Ge&K |
003 |
Nothia latissima (Grzybowski) |
004 |
Nothia robusta (Grzybowski) |
059 |
Paratrochamminoides acervulatus (Grzybowski) |
060 |
Paratrochamminoides deflexiformis (Noth), em. K&G |
061 |
Paratrochamminoides gorayskii (Grzybowski), em. K&Ge |
062 |
Paratrochamminoides heteromorphus (Grzybowski) |
063 |
Paratrochamminoides mitratus (Grzybowski) |
064 |
Paratrochamminoides olszewskii (Grzybowski) |
011 |
Placentammina placenta (Grzybowski), em. Ge |
120 |
Popovia beckmanni (Kaminski & Geroch) |
121 |
Popovia elegans (Kaminski) |
084 |
Praesphaerammina gerochi (Hanzlíková) |
085 |
Praesphaerammina subgaleata (Vasicek) |
037 |
Psamminopelta gradsteini Kaminski & Geroch |
006 |
Psammosiphonella cylindrica (Glaessner) |
005 |
Psammosiphonella discreta (Brady) |
008 |
Psammosphaera fusca Schultze, em. H-A&E |
009 |
Psammosphaera irregularis (Grzybowski), em. Li&Li |
048 |
Pseudonodosinella elongata (Grzybowski) |
049 |
Pseudonodosinella nodulosa (Brady), em. L&T |
094 |
Recurvoidella lamella (Grzybowski), em. Ch&J |
095 |
Recurvoides anormis Mjatliuk |
096 |
Recurvoides contortus Earland |
097 |
Recurvoides nucleolus (Grzybowski) |
098 |
Recurvoides retroseptus (Grzybowski), em. K&G |
099 |
Recurvoides setosus (Grzybowski) |
100 |
Recurvoides walteri (Grzybowski), em. M |
129 |
Remesella varians (Glaessner) |
050 |
Reophanus berggreni Gradstein & Kaminski |
051 |
Reophax duplex Grzybowski |
052 |
Reophax globosus Sliter |
053 |
Reophax pilulifer Brady |
054 |
Reophax subfusiformis Earland, em. H |
122 |
Reticulophragmium acutidorsatum (Hantken) |
123 |
Reticulophragmium amplectens (Grzybowski) |
124 |
Reticulophragmium garcilassoi (Frizzel) |
125 |
Reticulophragmium intermedium (Mjatliuk) |
126 |
Reticulophragmium pauperum (Chapman), em. Lu |
127 |
Reticulophragmium rotundidorsatum (Hantken) |
128 |
Reticulophragmoides jarvisi (Thalmann), em. G&K |
007 |
Rhabdammina linearis Brady |
031 |
Rzehakina epigona (Rzehak) |
032 |
Rzehakina fissistomata (Grzybowski) |
033 |
Rzehakina inclusa (Grzybowski) |
034 |
Rzehakina lata Cushman & Jarvis |
035 |
Rzehakina minima Cushman & Renz |
010 |
Saccammina grzybowskii (Schubert) |
086 |
Sculptobaculites barri Beckmann |
103 |
Spiroplectammina navarroana Cushman, em. G&K |
104 |
Spiroplectammina spectabilis (Grzybowski), em. K |
105 |
Spiroplectammina trinitatensis Cushman & Renz |
106 |
Spiroplectinella dentata (Alth) |
107 |
Spiroplectinella israelskyi (Hillebrandt) |
108 |
Spiroplectinella subhaeringensis (Grzybowski) |
036 |
Spirosigmoilinella compressa Matsunaga |
056 |
Subreophax pseudoscalaris (Samuel) |
055 |
Subreophax scalaris (Grzybowski) |
101 |
Thalmannammina subturbinata (Grzybowski), em. P |
065 |
Trochamminoides dubius (Grzybowski) |
066 |
Trochamminoides grzybowskii Kaminski & Geroch |
067 |
Trochamminoides proteus (Karrer), em. R |
068 |
Trochamminoides subcoronatus (Grzybowski) |
069 |
Trochamminoides variolarius (Grzybowski) |
112 |
Trochamminopsis altiformis (Cushman & Renz) |
Example of the taxonomic description:
ORIGINAL DESIGNATION: Reophax duplex var. α Grzybowski 1896.
TYPE REFERENCE: Grzybowski, J., 1896. Otwornice czerwonych iłów z Wadowic. Rozprawy Wydziału Matematyczno-Przyrodniczego, Akademia Umiejętności w Krakowie, serya 2, vol. 30, p. 276, pl. 8, figs. 23, 24. (not fig. 25).
TYPE SPECIMEN: Not originally designated. In their revision of Grzybowski's species from Wadowice, Liszka & Liszkova (1981) found the specimen corresponding to Grzybowski's original drawing (pl. 8, fig. 23) and designated it as the lectotype. This specimen is housed in the micropaleontological collections of the Jagiellonian University, Kraków (Grzybowski collection 115P, slide #15).
TYPE LEVEL: Campanian, red marls of the Subsilesian Unit of the Polish Carpathians.
TYPE LOCALITY: Wadowice, Poland. The lectotype locality is in the village of Zawada-Gaje, near Wadowice Poland (see remarks under H. dilatata).
DIAGNOSTIC FEATURES: Test relatively small, comprised of two (rarely three) embracing spherical chambers of almost equal dimension. Wall thick, made up of coarse, angular quartz grains, with a rough surface. Aperture a round opening, without a neck. Recent specimens are dark reddish brown in color.
SIZE: The lectotype is 1.1 mm in length. Recent specimens from the Hatteras Abyssal Plain average 0.4 mm.
SUSPECTED SYNONYMS: Reophax paraduplex Mjatliuk, 1970, Trudy VNIGRI, 282, p. 65, pl. 9, fig. 1a-3, pl. 10, fig. 2. [Paleogene, Ukrainian Carpathians].
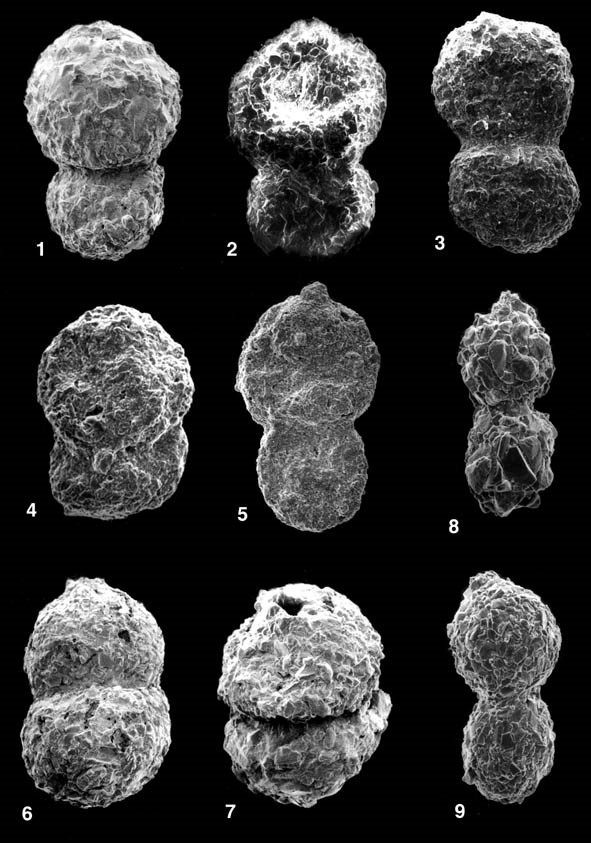
OBSERVED OCCURRENCES: Reophax duplex was originally described from Campanian red marls of the Subsilesian Unit of the Polish Carpathians (Grzybowski, 1896). It has subsequently been found in the Upper Cretaceous and Paleogene flysch deposits throughout the Alpine - Carpathian mountain chain. It was reported from the Upper Cretaceous and Paleocene of the Carpathians in Romania (Sandulescu, 1972), from the Paleocene and Eocene of the Carpathian flysch deposits in eastern Slovakia (Samuel, 1977), from the Maastrichtian of Austria (Grün et al., 1964), from the Danian in Bavaria (De Klasz & De Klasz, 1990), from the Paleocene to Eocene of the Polish Carpathians by Jurkiewicz (1967), and from the Senonian to Danian of the eastern Carpathians (Mjatliuk, 1970). Webb (1975) illustrated a specimen from the Paleocene of Hole 283, South Tasman Sea. Brohi (1994) illustrated a specimen from the Maastrichtian Mughal Kot Formation of the Khuzdar District in Pakistan. Preece (1999) reported it from the Miocene of Venezuela and Cabinda. Although not previously reported from the modern deep-sea environment, we have observed specimens that fit the description of R. duplex in box-core samples from the North Atlantic. Schröder (1986) illustrated a specimen as R. pilulifer from the Nova Scotian continental rise. Reophax duplex is a common component of flysch-type assemblages in the Atlantic and Tethys. We observed it in the Guayaguayare and Lizard Springs Formations, Maastrichtian to Early Eocene of Trinidad, in the Paleocene (Zones P1c - P5) of the flysch deposits in Zumaya Spain, and in the Maastrichtian to Paleocene of the Labrador Margin, in the Maastrichtian to Paleocene of the flysch sediments in northern Morocco, in the Upper Campanian to Maastrichtian of DSDP Sites 367 and 368, in the Paleocene Kamalapuram Formation of the Cauvery Basin, India, and in the Oligocene at ODP Site 1148 in the South China Sea. We did not observe R. duplex in Cretaceous abyssal assemblages or in benthic assemblages from deep-water limestones. In the modern deep sea, R. duplex occurs commonly on the Hatteras Abyssal Plain and on the Nova Scotian continental rise.

Figure 51-2a-d. Measured parameters of modern Reophax duplex from the Hatteras Abyssal Plain (n=33). Plots A - B are a measure of the size of the second chamber compared with the first chamber. Plot C measures the degree of roundness of the second chamber. Plot D measures the extent to which the chambers overlap.
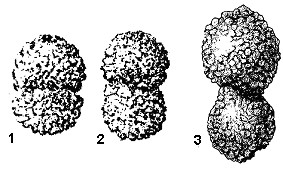
KNOWN STRATIGRAPHIC RANGE: Late Campanian to Recent.
BATHYMETRY: Cretaceous to Paleogene specimens are bathyal. Modern specimens are abyssal.
REMARKS: Grzybowski (1896) described two varieties of Reophax duplex, var. α and var. β. Liszka & Liszkowa (1981) regarded var. β to be a specimen of Reophax pilulifer. Our specimens of R. duplex from Trinidad are more finely agglutinated than specimens from the Upper Cretaceous of Poland. Modern specimens of R. duplex were found in gravity core-top samples from the Hatteras Abyssal Plain. Specimens from the Hatteras Abyssal Plain are smaller than the type specimens from Poland, which can be attributed to differences in water depth (bathyal vs. deep abyssal). Apart from differences in size, the overall shape characters of the modern specimens compare well with those of the holotype (Fig. 42-1). The relative proportions of the holotype (i.e., relative length of chambers, relative width of chambers) fit within the range of variation of the modern specimens. The only difference we determined is in the degree of overlap between the chambers. The amount of overlap of the chambers (measured by the width of the last chamber vs the width across the suture) is slightly greater in the holotype than in the modern specimens. However, this difference is relatively small, and we believe the modern specimens rightly belong in R. duplex.
ILLUSTRATIONS: Plate 51 - Reophax duplex Grzybowski Fig. 1. Paleocene, Szklary Poland, Skole Unit of the Polish Carpathians; Figs. 2-3. Eocene, Biecz Poland, Silesian Unit of the Polish Carpathians; Figs. 4-5. Paleocene, Lizard Springs Formation of Trinidad; Figs. 6-7. Paleocene, Labrador Margin, Roberval K-92 well, 2940'; Figs. 8-9. Recent, Hatteras Abyssal Plain, North Atlantic.